Baring Head, New Zealand
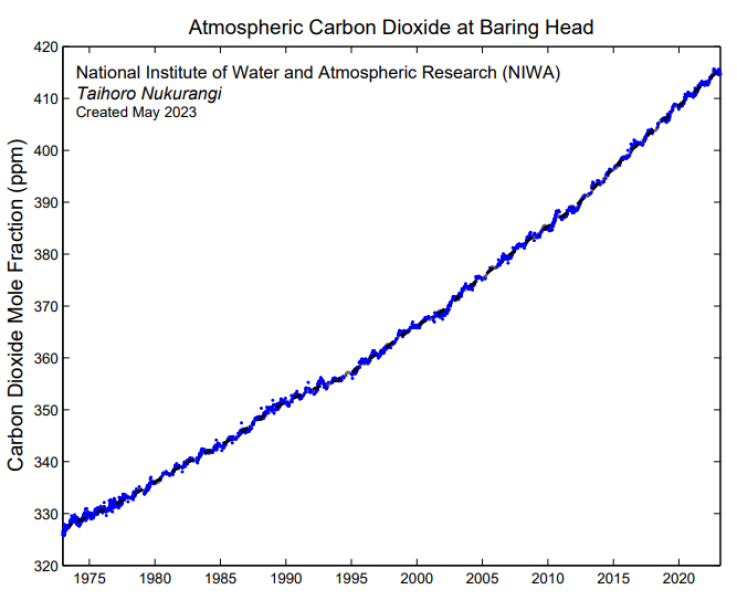
Atmospheric carbon dioxide (CO2) is measured continuously at Baring Head, providing the longest running record of this type in the southern hemisphere. Observations at this station were started in the early 1970s and continue to the present.
The dramatic rise in atmospheric CO2 over this period is due to human induced emissions from fossil fuel burning, land use change, and cement production. In addition to this trend, the atmospheric CO2 data reveals a seasonal cycle that is largely due to the effect of photosynthesis by plants, which take up more CO2 from the atmosphere during summer, with seasonality in the carbon exchange between the oceans and atmosphere playing a smaller role. The seasonal cycle observed at Baring Head is opposite the seasonal cycle observed by northern hemisphere sites such as Mauna Loa, because the seasons are reversed in the southern hemisphere, with summer occurring during December-March rather than June-Sept.
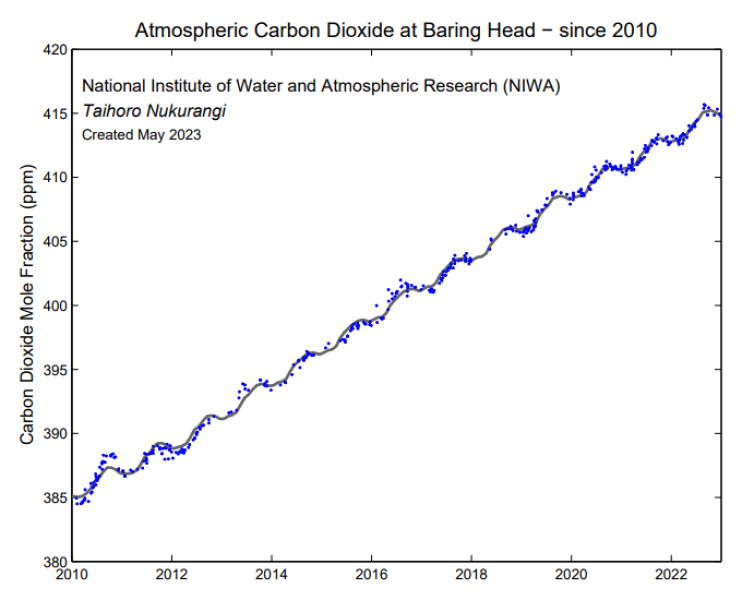
Air arriving at the station in southerly conditions that has not crossed land in recent days is plotted (blue points), these data provide a view of the background air from the mid-latitudes that is well mixed. The black line shows the smoothed curve with the seasonal cycle removed.
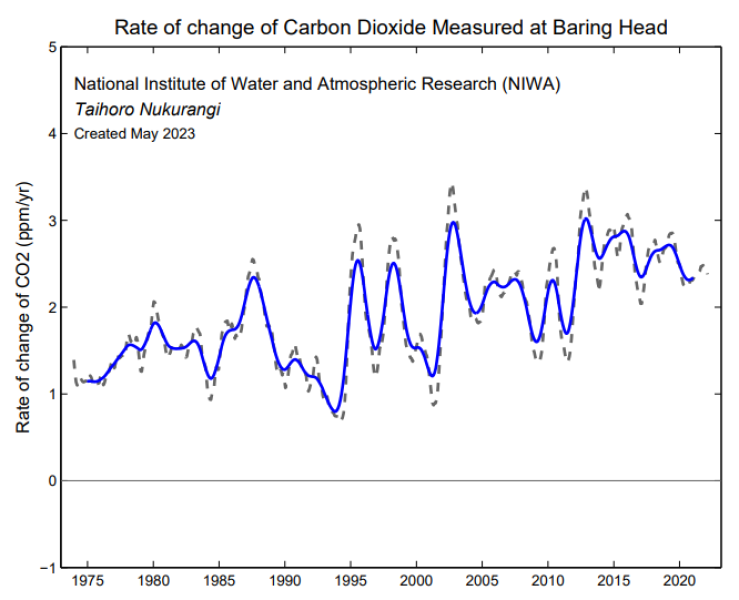
The growth rate of CO2 in the atmosphere calculated from monthly mean data that has had the seasonal cycle removed (grey). The blue line shows the growth rate after it has been smoothed with a 12-month running mean.
d13C in CO2
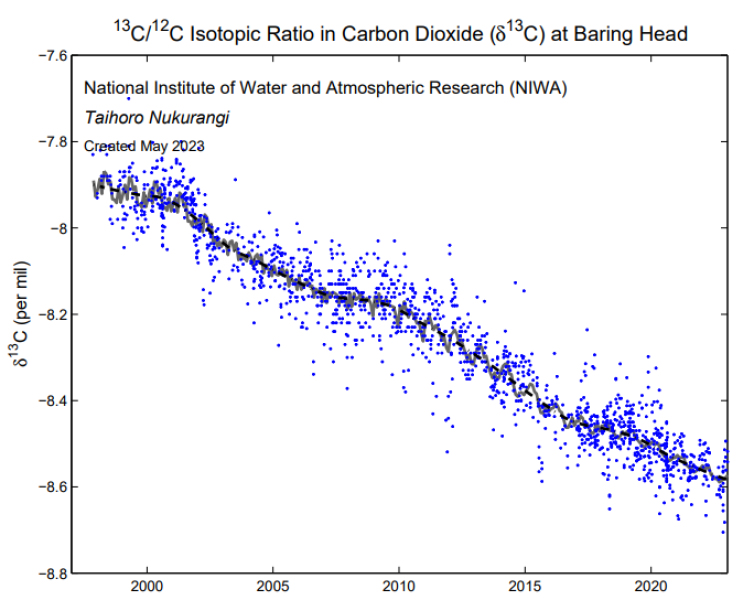
13C is a stable isotope of carbon (with one additional neutron compared to most carbon atoms). Many natural processes that produce or destroy CO2 influence the isotopic composition of atmospheric CO2. For example the 13C isotope is heavier than the normal form of carbon (12C), and plants tend to selectively assimilate the lighter isotopes during the photosynthetic process. In contrast, ocean exchange processes do not distinguish between 12C or 13C. This creates a difference in the relative ratio of terrestrial versus oceanic uptake of atmospheric carbon dioxide isotopes and allows scientists to use the 13C/12C isotopic ratio of CO2 to understand what processes are driving changes in the atmospheric CO2. This data set provides information about the role of oceanic and terestrial carbon pools. The 13C/12C isotope ratio in CO2 is shown as the relative difference from the VPDB* standard in permil (‰).
Individual flask measurements of CO2 at Baring Head (blue diamonds) have been fitted with a smoothed curve (gray line) using Seasonal Decomposition of Timeseries by Loess (STL). The dashed black line shows the smoothed curve with the seasonal cycle removed.
*VPDB = Vienna- PeeDee Belemnite standard, a standard originally obtained from a Cretaceous marine fossil, Belemnitella americana, from the PeeDee Formation in South Carolina. This material has a higher 13C/12C ratio than nearly all other natural carbon-based substances. For convenience it is assigned a delta 13C value of zero, giving almost all other naturally-occurring samples a negative delta value. The original sample was used up long ago, but a Vienna-based laboratory calibrated a new reference sample to the original fossil, giving rise to the widespread use of the term Vienna- PeeDee Belemnite standard, abbreviated to V-PDB.
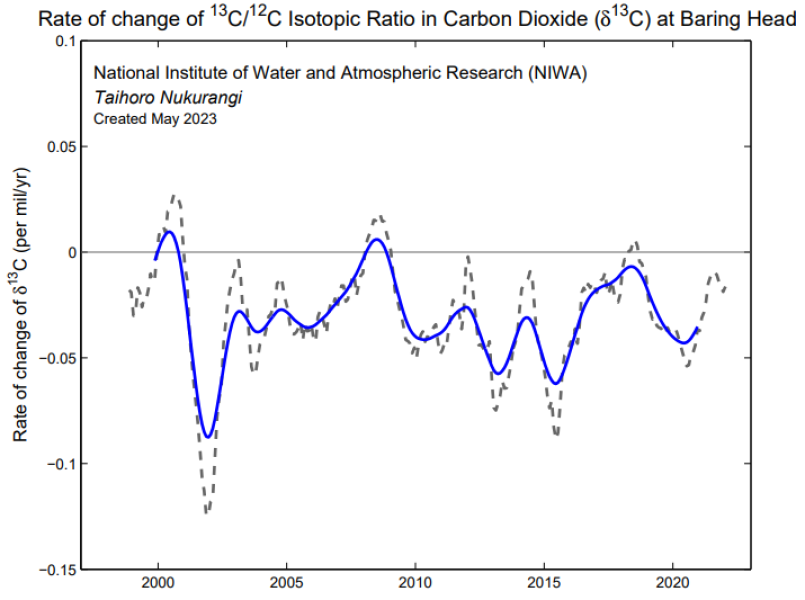
The relative change of 13C /12C in atmospheric CO2 calculated from monthly mean data that has had the seasonal cycle removed (grey). The blue line shows the growth rate after it has been smoothed with a 12 month running mean.
Arrival Heights
A secondary monitoring station is located at Arrival Heights, Antarctica. Similar trace gas measurements to Baring Head have been performed here though less frequently due to the logistical challenges in Antarctica.
The seasonal cycle ot atmospheric CO2 at Arrival Heights is similar to Baring Head and opposite to the seasonal cycle observed in the northern hemisphere.
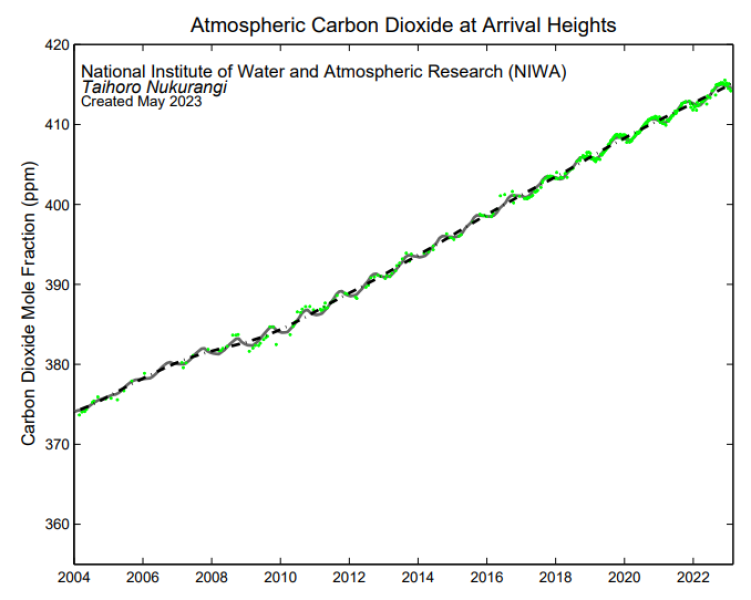
Individual flask measurements of CO2 at Arrival Heights (green diamonds) have been fitted with a smoothed curve (gray line) using Seasonal Decomposition of Timeseries by Loess (STL). The dashed black line shows the smoothed curve with the seasonal cycle removed.
Measurements of the stable carbon isotope 13C in atmospheric CO2 help scientists to distiguish terrestrial and oceanic processes that influence atmopsheric CO2 concentrations.
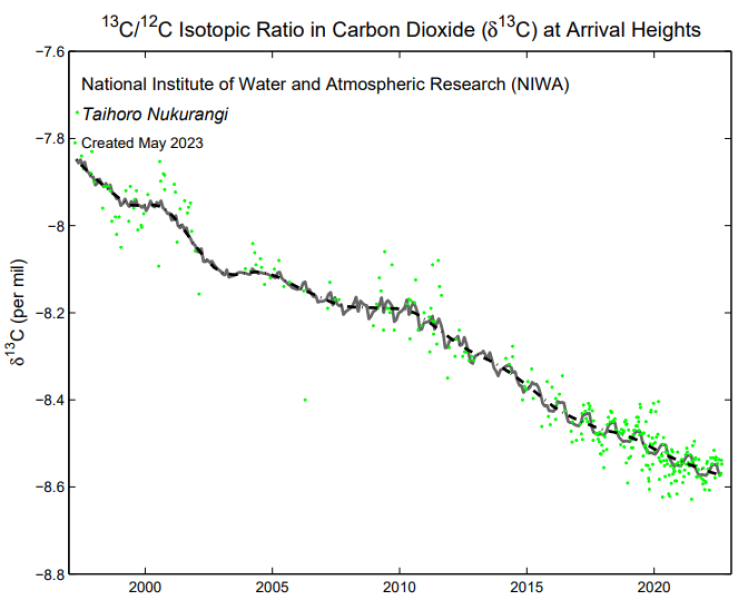
The 13C/12C isotope ratio in CO2 at Arrival Heights is shown as the relative difference from the VPDB* standard in permil (‰). Individual flask measurements of (green diamonds) have been fitted with a smoothed curve (gray line) using Seasonal Decomposition of Timeseries by Loess (STL). The dashed black line shows the smoothed curve with the seasonal cycle removed.
14C in CO2
14C, the radioactive isotope of carbon (with two additional neutrons compared to most carbon atoms), is a critical species for studying the interactions between the atmosphere, oceans, and land biosphere. It is produced in the atmosphere by solar radiation and nuclear activity. It is then absorbed into the oceans and land biosphere, where it is gradually removed by radioactive decay. This allows scientists to determine the residence time of carbon in plants and soils or the time that has passed since waters in the deep ocean have had contact with the surface.
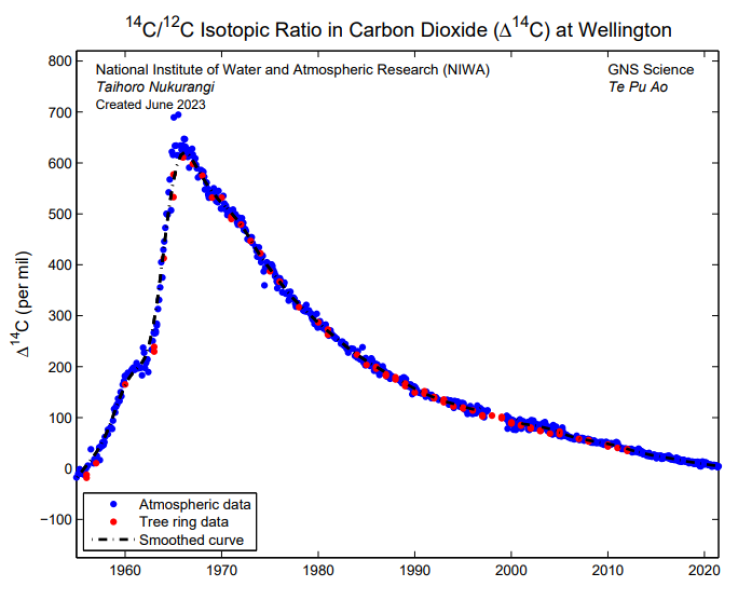
Observations of 14C/12C ratios in Wellington began in 1954, making it the longest running timeseries of atmospheric 14C in the world. The timeseries is dominated by a large peak in the mid 1960's, which is due to above ground testing of nuclear weapons. Since the nuclear test ban treaty in 1965, this peak has gradually decayed to near pre-bomb levels.
The 14C/12C ratio in atmospheric CO2. The blue data points represent individual samples collected over typically two-week periods at roughly monthly intervals, while the black line shows a smoothed curve fitted using Seasonal Decomposition of Timeseries by Loess (STL). The red data points represent a set of tree ring samples collected from two Kauri trees in Eastboune, Wellington. The more recent data are being measured using Accelerator Mass Spectrometry by the National Isotope Centre (NIC) at GNS Science, New Zealand, whereas early data used a proportional gas counting technique.