Ocean acidification – caused by increases in carbon dioxide in the atmosphere – is having detrimental effects on marine life globally. This feature investigates the causes of ocean acidification, its effect on New Zealand’s oceans and New Zealand’s efforts to monitor this complex issue.
The world’s oceans are acidifying as a result of the carbon dioxide (CO2) generated by humanity.
A report by the United Nations Convention on Biological Diversity last year noted that the ocean’s pH, a measure of acidity, had decreased by 26 per cent since the start of the industrial revolution, mirroring the proportion of manmade CO2 emissions that the oceans absorb from the air.
Globally the oceans’ average pH is currently 8.1, which is 0.1 lower than it was 250 years ago. This may not sound significant, but the pH scale is logarithmic, so a decrease of one pH unit represents a 10-fold increase in the acidity. What’s worse, this decline in pH is projected to continue in line with the increase in atmospheric CO2, leading to the most rapid decrease in ocean pH in the past 50 million years.
Ocean acidification, as this process is known, has happened before. CO2 from Siberian volcanoes caused the world’s oceans to acidify 252 million years ago, generating the greatest ever extinction of life on this planet.
A study published in Science earlier this year, led by Matthew Clarkson, a geochemist at the University of Otago, showed that the initial land-based extinction was followed by a second wave of marine extinction as ocean pH levels dropped by about 0.7 units.
This ancient calamity in the Permian era suggests that there is a lot at risk from a new onset of ocean acidification. Studies of global and New Zealand marine organisms suggest there will be a number of impacts, from the coast to the deep ocean. For example, NIWA scientists estimate that perhaps 25 per cent or less of the existing cold water coral locations around New Zealand will be able to sustain their growth by 2100 due to ocean acidification.
New Zealanders may also be more directly affected by ocean acidification. For example, shellfish aquaculture on the western coast of the United States has already been affected by reduced larval survival and weaker shells as a result of lower ocean pH.
Just this month the Minister for Primary Industries, Nathan Guy, launched a study by the New Zealand Institute of Economic Research, which showed that $276m in annual export earnings and 859 jobs are generated from aquaculture in Marlborough. To protect this industry, and the vulnerable marine ecosystems on which it relies, the New Zealand Government has funded a four-year research programme that includes cutting-edge monitoring and field experiments to identify how rapidly our coastal waters are acidifying and the likely effects on marine life.
Chemical changes in the ocean
The uptake of CO2 by the ocean is regulated by physical processes such as waves and temperature.
When CO2 is combined with water it forms H2CO3 (carbonic acid). H2CO3 breaks down rapidly into HCO3- (bicarbonate ions) and H+ ions (hydrogen ions).
The H+ ions combine with carbonate in seawater to form bicarbonate. This natural process has maintained the H+ ion concentration (and therefore the pH) of the ocean for thousands of years.
However, increasing CO2 emissions cause CO2 to enter the ocean at a faster rate, and the natural supply of carbonate (from coastal regions and the deep ocean) cannot keep pace. As a result there is an excess of H+ ions, and pH and carbonate are decreasing.
The resulting decrease in carbonate availability makes it difficult for many marine animals to maintain their calcium carbonate shells.
New Zealand is not immune
Cliff Law, NIWA’s Principal Scientist, Marine Biogeochemistry, says that a decade ago the focus on the effects of CO2 on climate masked the risks posed to the oceans.
“Back then scientists were interested in the oceans as a sink for CO2. Oceans were a key player in the models – absorbing a lot of humanity’s carbon emissions
“However, scientists started wondering about the effects of the extra carbon. We understand the oceans’ chemical dynamics, so it was a straightforward calculation to work out that adding more CO2 would lower the pH.” (See graphic, The Chemistry of Acidification.)
Scientists started looking for evidence of falling pH, but detecting the small year-by-year changes was difficult, requiring sensitive equipment and long-term monitoring to show clear trends. However, acidification is now apparent, with several time-series stations around the globe showing long-term trends of decreasing pH. For example, the pH at the Hawaii Ocean Time-series has fallen from 8.12 to 8.07 in the past 25 years.
It’s the same story elsewhere. Studies off the US west coast, a region critical to the oyster industry that experiences naturally low pH, recorded a decline in pH that has been linked to high larval mortalities in hatcheries.
A 2015 study by Oregon State University found that more than 80 per cent of respondents from the US’s west coast shellfish industry were convinced that acidification was having consequences, with 50 per cent of the industry reporting they had already experienced some impacts from acidification.
One operator, the Hog Island Oyster Company, has found that oysters brought in as juveniles from Oregon and Washington, grew less reliably and more slowly and had a higher mortality rate than they had several years earlier.
New Zealand is not immune to ocean acidification. The ‘Munida transect’ Time-series in sub-antarctic waters off Otago is the Southern Hemisphere’s longest-running record of pH measurements. Monitoring since 1998 has established a decline in pH that reflects the increase in atmospheric CO2 recorded at NIWA’s atmospheric research station near Wellington.
The Munida time-series is led by NIWA’s Kim Currie, in collaboration with the University of Otago's Department of Chemistry. Every two months she has collected water samples along a 65-kilometre line from the tip of Otago Harbour out to sub-antarctic waters. This time-series is particularly valuable because it covers both subtropical and sub-antarctic waters in a one-day trip, so is in a unique location.
During the transect University of Otago scientists measure the pH while Currie measures alkalinity, total dissolved inorganic carbon and CO2 as well as other related parameters. The supporting data help to determine what processes are causing changes in the properties of the water masses, including the changes in pH. For example, pH is linked to temperature and therefore varies between summer and winter and also year to year.
Currie refers to the time-series as “sentinel monitoring”.
“We have been taking regular samples in the open ocean for 17 years. The open ocean is less likely than coastal waters to be influenced by localised or short-term anomalies – so when we see change in the open ocean, we know something significant is going on.
“In fact it took over a decade before the series could discern a long-term change in pH from the natural variability.”
The New Zealand Ocean Acidification Observing Network (NZOA-ON)
Kim Currie is not the only one concerned by the acidification issue. She found many willing partners when she sought to set up a nationwide network of stations to monitor coastal pH.
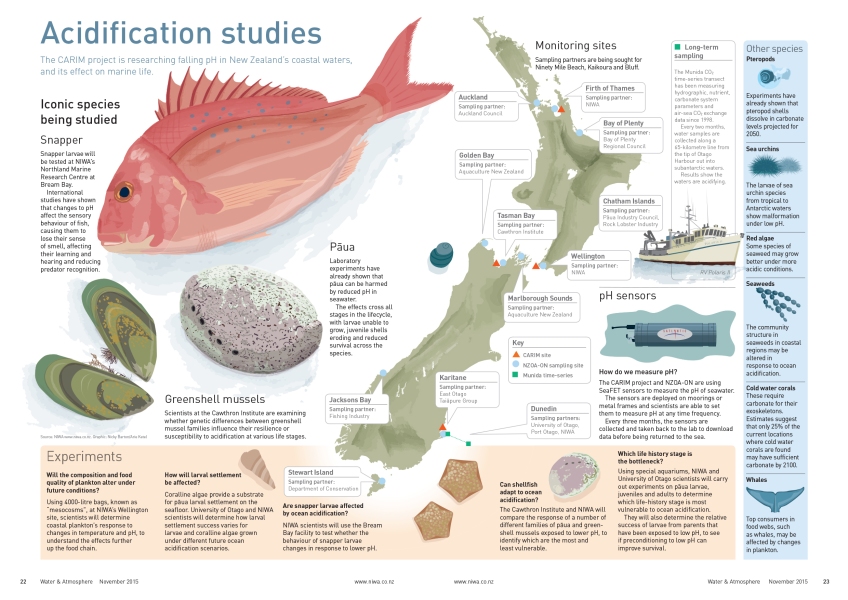
The New Zealand Ocean Acidification Observing Network (NZOA-ON) comprises 14 pristine and urban monitoring sites located around our shores.
In true Kiwi style the Network is actually the co-ordination of a wide range of sampling partners. The Network uses existing data collection infrastructures where possible, for example three NIWA sites, two sites monitored by regional councils, and a site on Stewart Island monitored by the Department of Conservation.
It has been expanded to include others, such as a pāua operation in the Chatham Islands and a fishing company on the West Coast, which have agreed to take samples at the location of their operations.
The NZOA-ON sampling partners collect water samples, liaise on shipment and logistics, and assist with the deployment of the sensors. Fortnightly water samples from each site are sent to NIWA’s Dunedin laboratory for the analysis of ocean acidification parameters – alkalinity and total dissolved inorganic carbon - which allow the calculation of pH and carbonate availability. pH sensors are also deployed for four or five months at each site on a rotating schedule, to measure the short-term pH variability at each location.
Data gathered from sampling is used to determine local conditions and to provide a baseline against which to assess future change. The Network is linked to the Global Ocean Acidification Observing Network, an international initiative led by the National Oceanic and Atmospheric Administration in the US, which co-ordinates global monitoring.
Currie says a decade of data may be needed to assess reliably the variability of coastal pH and whether the level is changing.
“The first two years will give us a baseline picture of the variations in pH of our coastal waters, but we’ll need longer to know whether it’s declining,” she says.
What’s the worry?
There are no New Zealand data on public awareness of the problem, but international studies show only low levels of alarm. Oregon State University estimated the public awareness of acidification and its impacts at about 20 per cent, compared with widespread awareness from those involved in the commercial fishing industry.
A 2014 United Kingdom study also found that only 20 per cent of the public had ever heard of ocean acidification.
In New Zealand, Anna Crosbie, Senior Aquaculture Analyst at the Ministry for Primary Industries, says that at the moment ocean acidification is not affecting the domestic aquaculture industry.
“However, it is important that we learn the lessons from other ocean acidification events around the globe, and improve our understanding of adaptive measures we can take to improve our long-term resilience to changing ocean chemistry.”
Mary Sewell, Associate Professor of Biology at the University of Auckland, says ocean acidification has the potential to affect food security and other ecosystem services that are provided by the oceans.
Miles Lamare, from the University of Otago’s Department of Marine Science, says laboratory experiments have already shown that abalone, including New Zealand pāua, can be harmed by reduced seawater pH.
The effects are seen at all stages in the lifecycle, such as larvae not being able to grow shells or develop normally and juveniles having reduced growth, thinner and eroded shells, and reduced survival rates.
Operators in the New Zealand pāua industry are worried that they are seeing the first signs of decreasing pH in the seas around New Zealand.
Jeremy Cooper, Chief Executive of the Pāua Industry Council, believes it’s only a matter of time before the industry has to face up to major problems.
“Our divers are working at the coalface and are completely in tune with reality. Many people think it will be the next generation’s problem, but we know this is not the case as divers are seeing changing ecosystems. When pāua shells and other shellfish begin dissolving in the water we will know we have a real problem.”
Lessons have already been learned from the Whiskey Creek Shellfish Hatchery in Oregon, US. Its operators discovered that seawater of lower pH inhibited shell growth, and oyster larvae were not surviving beyond 48 hours. The larvae were simply unable to form the first layer of calcium carbonate needed for survival.
Cooper points to NIWA research that suggests pāua will likely suffer the same fate if ocean acidification continues.
“For our industry, if juvenile pāua do not survive, the fisheries’ replenishment pathways will be completely disrupted and the resource will begin a downward spiral. If this happens it will be only a matter of time before pāua essentially becomes a mined resource, unable to replenish itself.”
A collaborative effort to tackle coastal acidification in New Zealand
Scientists are now working with stakeholders from industry, ministries, regional councils and the Department of Conservation, and also iwi partners, to combine resources to tackle coastal acidification under one banner – a four-year programme funded with $4.9m from the Ministry of Business, Innovation and Employment (MBIE).
This project, dubbed CARIM (Coastal Acidification: Rate, Impacts and Management), is co-ordinated and led by NIWA and is designed to establish the scale of acidification and how it is affecting New Zealand coastal ecosystems. It effectively knits together existing frameworks with new research directions and collaborations to enable scientific breakthroughs into the impacts and mitigation of coastal acidification.
Rob Murdoch, NIWA’s General Manager of Research, says ocean acidification is particularly concerning in coastal regions where New Zealanders access most seafood, and where reductions in pH can be made worse by land runoff.
Cliff Law, the CARIM programme leader, says pH monitoring will take place at the Firth of Thames, Karitane and Nelson Bays – three locations that are important for iconic species including GreenshellTM Mussels, pāua and snapper, which are ecologically, economically and culturally important in New Zealand.
“We will determine the sensitivity of their different life stages to lower pH, and also whether associated changes in food quality, availability and habitat will affect their survival. We will also examine the potential for shellfish to adapt to acidification, just one of the tools and approaches that may mitigate the impacts.”
After years of small and disparate scientific efforts, Kim Currie is delighted.
“The study of ocean acidification is finally starting to take off in New Zealand. The previously unco-ordinated monitoring and independent impact studies are now coalescing into a unified effort.”
Where and how much?
Law says MBIE is enabling the first coherent, ‘end-to-end’ project on ocean acidification.
“The separate components within CARIM are interlinked; we’re measuring the pH changes, the causes and the impacts on marine life, and identifying how to respond.
“The overall result will be better models, allowing more accurate predictions of the impacts of acidification in coastal waters, and providing management options for stakeholders.”
Law is particularly pleased with the focus on the measurement of coastal pH levels.
“Coastal waters are the most variable in their natural pH levels; they are where we get the most benefits in terms of food, recreation and other amenities, yet also where we affect the ocean most.”
The data will help to fine-tune models of pH in coastal waters, and also shellfish population models. They will also give clues as to how to use the natural resilience of shellfish to adapt, which may benefit aquaculture.
The Firth of Thames study will use nutrient, current and river flow data in dynamic models to assess the relative contributions of the atmosphere, land and ocean to changes in pH, informing management options for reducing the impacts of land-based activities on coastal pH.
The impacts of acidification on phytoplankton
Law will co-ordinate research into the impacts of acidification on phytoplankton during CARIM.
At NIWA’s Greta Point facility a number of 4000-litre tubes – known as ‘mesocosms’ – will be filled with surface water from Wellington Harbour. The objective is to see how plankton in the water respond to the changes in temperature and pH that are projected for this region in the future.
Permeable tubing regulates the diffusion of CO2 into water in the mesocosms under the control of an automated feedback system, allowing the researchers to set pH and temperatures to future levels.
“Plankton are responsible for at least half of the oceans’ CO2 uptake, so they help to regulate climate. Just as importantly they form the base of the marine food web. Understanding their response to acidification is critical to both climate models and the future productivity of our fisheries and ecosystems.”
In one of the buildings near the mesocosms, another CARIM experiment will look at the sensitivity of pāua to temperature and pH. This world-class facility consists of rows of pāua-filled aquariums festooned with equipment to control and monitor their watery environment.
These experiments, co-ordinated by NIWA’s Vonda Cummings, are examining the effects of combinations of pH and temperature on different life stages of pāua – particularly the settlement of the larvae and mortality rates, where problems have been observed in other parts of the world.
In combination with a Ministry for Primary Industries project, and in collaboration with Australian scientists, CARIM is also examining the effects of lower pH on snapper larvae.
International studies have shown that low pH affects the sensory behaviour of fish, causing them to lose their sense of smell, wander farther from safety and lose the capacity for predator recognition. These impacts may have big implications for snapper survival, and the studies to be carried out by Darren Stevens at NIWA’s Bream Bay facility are important to establishing the future prospects of one of the nation’s favourite fish.
Monitoring shellfish
Until now the impacts of falling pH have been mainly theoretical and laboratory based.
The University of Otago’s Miles Lamare says we cannot know the effects of falling pH until we extrapolate the laboratory findings to the real world.
“Ocean acidification will coincide with a number of other key environmental changes, such as ocean warming and coastal degradation,” says Lamare.
“Laboratory experiments probably don’t realistically replicate the variability that occurs in the natural world both in time and between habitats. Nor have short-term laboratory experiments been able to take into account the capacity of marine species to adapt and acclimate to pH changes.”
Lamare’s own experiment will look at settlement from the plankton onto the seafloor, a key stage in the lifecycles of marine invertebrates like mussels and pāua.
“Settlement involves specific cues that ensure animals settle in suitable locations, so we need to understand if this process is affected by ocean acidification.
“We will be growing suitable substrates under different future ocean acidification scenarios, and will examine how settlement behaviour changes. To understand patterns in settlement under realistic ocean acidification scenarios, we will quantify the changes in substrates by examining differences in the microbial community and biofilms.
“We will rear larvae under the same pH conditions to explore whether changes in settlement can be a result of changes in larvae development, sensory capacity and behaviour. For example, larvae seem to develop more slowly in low pH. Work on other invertebrate larvae suggest they become less selective about where they settle if their development is slower than normal.”
Lamare also hopes to explore the processes in a range of marine invertebrates to see if these issues are species specific or general, and if mussels and pāua are more or less sensitive at the settlement stage. This would be essential information for a shellfish industry considering its future farming stock.
At the end of the four years, says Lamare, the lab and field studies will be incorporated into pāua population models, and data on natural variability in the coastal pH environment will help to create more accurate lab experiments.
Norman Ragg, of the Cawthron Institute, leads the CARIM experiments looking at physiological differences between mussels that are resilient and those that are susceptible to acidification.
“Our initial task is to place sentinel mussels at the three sites where pH is being monitored. These will be babies straight out of the hatchery, around the size of a grain of rice, which will act as bio-sensors.
“During year one of the project we will create a single ocean acidification scenario or environment in the laboratory using NIWA projections. We will then monitor 40-80 new families and examine how they respond to these conditions. The aim is to determine vulnerability in the different life stages, and identify those families showing more resilience or susceptibility.”
Ragg says the most resilient mussel families could be a great commercial tool for an acidified future.
“By year two we want to be able to identify the special biological material in the families at the extreme ends of the scale. What is it that brings resilience into the species at a biological level? Are the mothers’ experiences, such as exposure to low pH, conferring benefits? If so, does this show up in the young as resilience?”
He says there is certainly no clear evidence of mussels being affected by ocean acidification at this stage.
“It’s important to clarify the difference between ocean acidification and coastal acidification. CO2 from the air is not the only contributor to coastal acidification.
“At this stage we have little understanding of how local inputs such as river flow, local land use such as forestry and nutrient runoff might be affecting our coastal waters – and the places we are farming mussels.”
Ragg says the variables in the processes make for complex science.
“Nitrate runoff from fertilisers contributes to algae growth, which is good for mussels. On the other hand, when the algae start dying off in late summer and autumn, the associated decomposition could increase CO2 and decrease the pH in water bodies like the Firth of Thames.”
The University of Auckland’s Mary Sewell is working with Cawthron on the impacts of ocean acidification on the early development of mussels.
“We are measuring changes in gene expression, protein expression and small chemical molecules to identify biomarkers of coastal acidification stress.
“This will allow us to understand better the biological functions that are being affected by ocean acidification and the potential impacts that this might have in the coastal marine ecosystem.”
A major benefit of the CARIM project is that monitoring of shellfish can be matched to the monitoring of coastal pH on a daily and seasonal basis, and also spatially at the sentinel sites.
“This will provide a huge leap forward in understanding how ocean acidification will affect New Zealand’s coastal marine ecosystems,” Sewell says.
“We can design our experiments to measure the responses of mussels and pāua to ocean acidification in current and future conditions, then use these results in predictive models.”
The experiments will provide clues to the impacts of ocean acidification in uniquely New Zealand conditions – such as on aquaculture in shallow embayments where there is sometimes significant nutrient runoff from land. In these areas the combination of increased atmospheric CO2 and coastal nutrient enrichment can result in a very-low-pH environment.
“New Zealand needs to understand these in detail, so that we can develop strategies to mitigate the effects of ocean acidification.”
Soluble solutions
While one thread of the CARIM project measures the extent and impacts of acidification, the other will be drawing lessons on adaptations and solutions.
Jeremy Cooper says if the effects of ocean acidification on pāua become real, pāua businesses may become more like farming operations, hatching larvae in controlled conditions and allowing them to strengthen their shells before reseeding them into the ocean.
“We need to leapfrog Mother Nature’s bottleneck. Instead of worrying, we need to identify the problems and work with them.
“It’s highly likely that ocean acidification will affect different parts of the coastline at different rates. If we can identify areas of ocean around New Zealand where ocean acidification is not having as great an effect, we could target these areas for enhancement to maximise overall productivity.”
He thinks the solution also requires a wider view of the inputs and outputs, to see where humanity can help alter conditions.
“Mussel shells are largely made up of calcium carbonate, but every year tonnes and tonnes of their calcium carbonate shells are being pulled out of the ocean and end up in landfills. This could be compounding the problem. In other countries around the world they have to return shellfish shells to the ocean to help replenish the micro and macro nutrients in the water column. Perhaps we need to be looking at this as well.”
Miles Lamare is hopeful that CARIM will provide greater information about the capacity of coastal species to adapt to a changing environment.
“With good information, worry about ocean acidification might be replaced with the positive management of any issues.
“We may be able to implement strategies that mitigate future problems. For example, we could decide to pursue farming resilient strains of mussels, or restoring resilience to changes in coastal ecosystems by improving coastal health.”
Cliff Law says the jury is out on how we can adapt our use of the ocean to acidification, but New Zealand is about to make a valuable contribution to the emerging acidification science.
“The CARIM project will provide more coherent models that include both direct and indirect ecosystem effects to obtain a clearer picture of how coastal acidification will affect these iconic species, and how we can manage those effects.”
Every three years ocean acidification scientists converge for the International Symposium on the Ocean in a High CO2 World. When it is held next May in Tasmania, Law will be explaining the plans and progress of the CARIM project.
After at first failing to appreciate the effects of CO2 on oceans, the world has a lot of catching up to do.
Surveying the shellfish industry
A 2015 US survey by Oregon State University found that more than 80 per cent of respondents from the West Coast shellfish industry were convinced that acidification was already having consequences.
About half of the respondents reported experiencing the impacts in their work, with 97 per cent saying ocean acidification had caused them financial damage.
Rebecca Mabardy, the lead author on the study, says the shellfish industry recognised the consequences to a far greater extent than the public.
Marine ecologists at Oregon State University have noted that many shellfish farmers have seen the effects of acidified water on the survival of juvenile oysters. But some early success in hatcheries’ adaptation to changing conditions has generated guarded optimism that the industry can adapt.
Further information
NIWA science delves into ocean acidification
Video: ocean acidification - what does it mean for shellfish?
Note: This feature originally appeared in Water & Atmosphere, November 2015
Related links
- Read Water & Atmosphere Issue 14, November 2015
- Read more coasts and oceans news
- New Zealand Ocean Acidification Observing Network (NZOA-ON)