PDF of this article (247 KB)

Stephen Wood, Dave Lowe, Brian Connor, Karin Kreher, Sylvia Nichol and Greg Bodeker
Precision instruments and extensive international and local collaboration are the hallmarks of NIWA’s atmospheric research in Antarctica.
Ozone depletion and increasing greenhouse gases both feature regularly in the news media these days and the connection between ozone and Antarctica is well known. We can also learn much about greenhouse gas concentrations from studies in the Antarctic. NIWA’s atmospheric research in Antarctica is aimed mainly at understanding these two important issues. Because the atmosphere has no national boundaries, the research involves scientists from many countries.
Ozone
The ozone depletion that occurs over Antarctica each spring – the Antarctic ozone hole – is a striking example of human influence on the atmosphere. In the space of a few weeks most of the ozone in the lower stratosphere over Antarctica disappears. This is due to a remarkable combination of dynamics and chemistry in the cold Antarctic atmosphere that makes industrial chemicals far more effective in depleting ozone (see “The 2000 Antarctic ozone hole”).

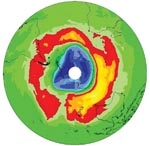
In the 1980s international action resulted in a treaty – the Montreal Protocol – to limit and ban the chemicals responsible for the damage. Although the Montreal Protocol is now proving effective, it will be decades before some of these gases recover to pre-ozone-hole concentrations. Over the past few years the ozone hole has varied considerably in size and severity (see also “NIWA’s southernmost technician and the 2002 ozone hole”). To confirm the predicted ozone recovery, we need to demonstrate a consistent decrease in the extent and intensity of the ozone hole over many years.
Greenhouse gases
Although it is widely accepted that increases in greenhouse gases in the atmosphere are causing the Earth’s surface to warm on a global scale, we still do not fully understand where the gases come from and where they end up. Carbon dioxide (CO2), the most important greenhouse gas, has increased by more than 30% since preindustrial times (see Water & Atmosphere 8(1): 14–16). We know that about half of the CO2 emissions remain in the atmosphere, enhancing the Earth’s natural greenhouse effect, and that the remainder probably gets incorporated into land-based and ocean ecosystems. But how much goes into each of these reservoirs?
The Southern Ocean is thought to be the world’s largest sink of carbon, but we still have to determine how large. As a step towards quantifying this, NIWA has made high-precision stable isotope measurements of atmospheric CO2 and oxygen in the Antarctic (Water & Atmosphere 4(2): 11–12, 5(2): 16–17). The differences in stable isotope levels can be used to estimate how much CO2 is absorbed by the southern oceans. Early results show a significant effect of ocean circulation and upwelling events on atmospheric CO2 and O2 concentrations. This project is part of an ongoing international research effort to work out the proportions of carbon from human activities absorbed by the oceans and by the land.
Within another international programme, NIWA is investigating changes in the isotope composition of greenhouse gases like methane and CO2 (see “Antactic ice: the world’s air museum”). We know that the atmosphere is changing quickly. A key benchmark is the composition of the atmosphere before human activity dramatically changed it.
When snow falls it traps air; over time, as the snow turns to ice, the trapped air remains as tiny bubbles within the ice. The deeper the bubble in the ice, the older is the air within it. The ice sheets in Antarctica thus provide a remarkable museum of the chemical state of the atmosphere extending over millennia. For example, the naturally occurring radioactive isotope carbon-14 in methane trapped in these bubbles can tell us whether the gas came from fossil fuels or from natural sources like swamps. Initial results show that 60 years ago up to 15% of atmospheric methane was derived from fossil sources. Higher percentages imply a larger industrial component in sources of atmospheric methane.
Gas measurements and modelling
To measure atmospheric trace gases, especially those in the stratosphere, we rely on the fact that different gases absorb light in different parts of the spectrum. We use specialised ground-based equipment to measure quantities of ozone and several other gases active in ozone depletion chemistry, such as HCl, ClO and BrO. These instruments can also be used to determine greenhouse gases and other gases in the troposphere, such as the products of fires in the tropics.

Most of our measurements use sunlight, though it is possible to use moonlight for some measurements in the dark winter months. A perennial task is to refine our measurement and analysis techniques to improve the information retrieved (see Bromine on Ice panel below).
We also measure gas concentrations directly from air samples collected both in Antarctica and during transport flights between Christchurch and McMurdo.
To interpret the measurements of stratospheric gases, we have developed a model that tracks how stratospheric chemistry changes under given conditions. We plan to use the model to separate the effects of gas movement from chemical changes in a series of measurements of trace gases made at regular intervals over time at a single site. A separate modelling project has looked at the effect of spring-time ozone depletion on places at mid-latitudes. The work has shown that more than half of the observed summer time reduction in ozone over New Zealand since pre-ozone-hole times is due to the dilution effect when annual ozone holes dissipate, leading to significant increases in UV over New Zealand in the past two decades (see also Water & Atmosphere 7(4): 7–8).
NIWA will continue to recognise the importance of the Antarctic atmosphere in the global system with a strong measurement programme, backed up with modelling and analytical research and increased collaboration with overseas researchers.
Bromine on Ice
Investigating the future of the Antarctic ozone hole brought a PhD student from the University of Auckland to Scott Base for the 2002 season. Robyn Schofield, a University of Otago honours graduate and FRST Bright Futures Scholarship recipient, has pioneered a new measurement technique in partnership with NIWA scientists and successfully applied it to bromine monoxide, BrO.

Knowing how much bromine is in the stratosphere is critical to predicting the future severity of the ozone hole. Chlorine-containing gases (which derive from breakdown of CFCs) are controlled under international agreements and rapid decreases are expected. However, bromine-containing gases also play a major role in ozone loss, and will decline more slowly. The more bromine in the stratosphere, the longer the ozone hole will persist.
For some years NIWA has been measuring total volumes in the atmosphere of BrO, nitrogen dioxide (NO2), and chlorine dioxide (OClO), all of which are involved in ozone depletion. We have now developed a “direct-sun” spectrometer to separate tropospheric from stratospheric information. The new technique combines two different viewing methods with a computer model developed by Robyn. In the first method, looking directly at the sun results in a long light path through the troposphere; in the second, looking at scattered light from the zenith produces a similar enhancement in the stratosphere.
After successfully applying the new technique to BrO measurements at Lauder (45 °S), Robyn went on campaign in the Antarctic. She operated a direct-sun instrument from early September to late October every clear morning and evening. Robyn then used these observations to measure stratospheric and tropospheric BrO.
A high variability was observed for the stratosphere, partly because of the unusual stratospheric warming in spring 2002. Robyn measured a background concentration of 0.3 parts per trillion (ppt) BrO in the troposphere. On 24 and 25 October 2002 there was a “bromine explosion”, equivalent to 7 ppt BrO in the lower 2 km of the atmosphere. First measured in the mid 1990s, these events are thought to result from an accumulation of bromine- and chlorine-containing sea salt on the winter ice pack, leading to a rapid release of bromine and consequent severe ozone depletion close to the ground.
Teachers: this article can be used for NCEA Achievement Standards in Geography (1.1, 1.6, 1.7, 2.1, 2.6, 2.7, 3.1, 3.6, 3.7), C&S (1.2), Physics (1.2, 2.2, 2.7). See other curriculum connections at www.niwa.co.nz/pubs/wa/resources
Stephen Wood, Brian Connor, Karin Kreher and Greg Bodeker are based at NIWA, Lauder; Dave Lowe and Sylvia Nichol are at NIWA in Wellington.
Acknowledgements This work is supported by the FRST programme “Drivers and Mitigation of Global Change” under contract C01X0204 and relies on technical and logistic support from Antarctica New Zealand. It has strong collaboration with overseas groups such as the Universities of Heidelberg and Denver, The State University of New York, NOAA in Boulder, CSIRO in Melbourne, and the Scripps Institution of Oceanography in California. The programme benefits from joint work with universities in a range of PhD students: Rona Thompson (Victoria University); Rebecca Batchelor (University of Canterbury); Jelena Ajtic (University of Canterbury); and Robyn Schofield (University of Auckland).
